Singapore has taken a major step forward in advancing artificial intelligence in healthcare with the regulatory approval of CystoSmart, an AI-powered software designed to assist in the detection of bladder tumors. The clearance by Singapore’s Health Sciences Authority (HSA) reflects the country’s growing commitment to adopting innovative medical technologies that improve diagnostic accuracy and patient outcomes.
This approval marks a significant milestone not only for bladder cancer care but also for the broader adoption of AI-enabled diagnostic tools in clinical practice.
Bladder Cancer: A Persistent Diagnostic Challenge
Bladder cancer is one of the most commonly diagnosed cancers worldwide and poses a unique challenge due to its high recurrence rate. Many patients require repeated surveillance over several years, placing a heavy burden on both healthcare systems and patients.
White light cystoscopy remains the standard diagnostic method for identifying bladder tumors. During this procedure, clinicians visually examine the bladder using an endoscopic camera. However, despite its widespread use, conventional cystoscopy can miss a notable percentage of tumors, particularly small or flat lesions that are difficult to distinguish from healthy tissue.
These limitations highlight the need for advanced tools that can assist clinicians in detecting subtle abnormalities more reliably.
What Is CystoSmart and How Does It Work?
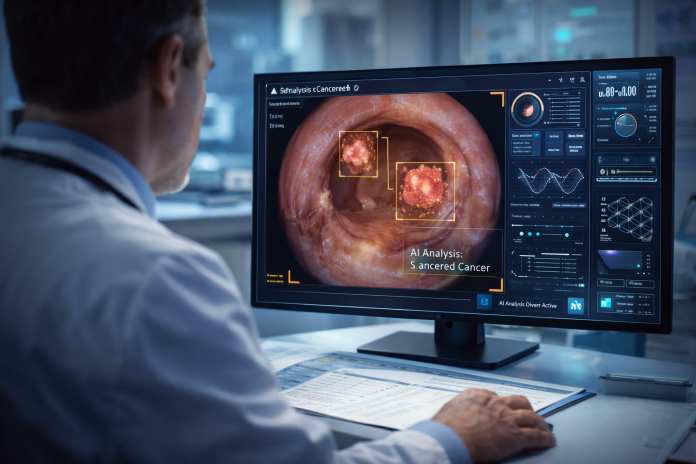
CystoSmart is an AI-based software tool developed to enhance bladder tumor detection during cystoscopy procedures. It uses advanced machine-learning algorithms to analyze real-time video feeds and images captured during examinations.
The software assists clinicians by identifying areas of concern and highlighting potential tumors that may otherwise go unnoticed. Importantly, CystoSmart does not replace existing diagnostic workflows. Instead, it works alongside standard white light cystoscopy, acting as a clinical decision-support tool.
CystoSmart is compatible with both flexible and rigid cystoscopes and can be used with reusable as well as single-use scopes. It can operate during live procedures and also supports post-procedure image analysis, allowing clinicians to review findings more thoroughly.
Regulatory Approval and Its Significance
The approval by Singapore’s Health Sciences Authority is particularly noteworthy due to the country’s stringent regulatory standards for medical devices and software. Gaining clearance indicates that CystoSmart meets high benchmarks for safety, reliability, and clinical performance.
This approval also strengthens Singapore’s position as a leading hub for digital health innovation in Asia. By supporting AI-driven medical solutions, the country is encouraging the integration of technology into everyday clinical practice while maintaining patient safety as a top priority.
Additionally, such regulatory clearances often serve as reference points for other international markets, potentially accelerating adoption in multiple regions.
Clinical Performance and Accuracy
One of the strongest advantages of CystoSmart lies in its clinical performance. According to validation data reviewed during the approval process, the AI software demonstrated exceptionally high sensitivity and specificity in detecting bladder tumors.
High sensitivity means the tool is highly effective at identifying true tumor cases, while high specificity reduces the likelihood of false positives. Together, these metrics help clinicians make more confident decisions during diagnosis and follow-up care.
Improved accuracy can lead to earlier detection, more precise treatment planning, and better long-term outcomes for patients.
Benefits of AI-Powered Bladder Cancer Detection
The integration of AI tools like CystoSmart into urology practice offers several important benefits:
1.Improved Early Detection
AI algorithms can recognize visual patterns that are difficult for the human eye to consistently detect, helping identify tumors at earlier stages.
2.Reduced Miss Rates
By acting as a second set of eyes, AI reduces the risk of overlooked lesions during cystoscopy.
3.Enhanced Clinical Confidence
AI-assisted insights support clinicians in making informed diagnostic and treatment decisions.
4.Better Surveillance Outcomes
Given the high recurrence rate of bladder cancer, accurate and consistent detection during follow-up examinations is crucial.
5.Workflow Efficiency
AI support can reduce cognitive fatigue during long procedures, helping clinicians maintain focus and efficiency.
AI in Healthcare: A Growing Trend
The approval of CystoSmart aligns with a broader global trend toward adopting AI in healthcare. From radiology and pathology to gastroenterology and oncology, AI is increasingly used to enhance diagnostic precision and reduce variability in clinical outcomes.
Singapore has been particularly proactive in creating a supportive ecosystem for medical AI. Investments in digital health infrastructure, regulatory innovation, and clinical validation frameworks have made it an attractive environment for developing and deploying advanced healthcare technologies.
Impact on Patients and Healthcare Systems
For patients, AI-enabled diagnostic tools can mean faster, more accurate diagnoses and potentially fewer repeat procedures. Early and reliable detection also improves treatment planning and can reduce the physical and emotional stress associated with prolonged uncertainty.
For healthcare systems, tools like CystoSmart offer scalability. Since the software integrates with existing cystoscopy equipment, hospitals can adopt it without major infrastructure changes. Over time, improved detection accuracy may also help reduce long-term treatment costs associated with advanced or recurrent disease.
Future Outlook for AI in Cancer Detection
The approval of CystoSmart represents more than just a single product milestone. It reflects a shift toward AI-augmented medicine, where technology enhances human expertise rather than replacing it.
As AI models continue to evolve, similar tools are expected to expand into other cancer types and diagnostic procedures. Ongoing clinical research, real-world evidence collection, and regulatory collaboration will be key to ensuring these technologies are deployed responsibly and effectively.
Conclusion
Singapore’s approval of the AI software tool CystoSmart marks an important advancement in bladder cancer detection and AI-driven healthcare innovation. By improving diagnostic accuracy and supporting clinicians during cystoscopy procedures, the technology has the potential to significantly enhance patient outcomes.
As AI continues to reshape modern medicine, solutions like CystoSmart demonstrate how thoughtfully designed technology can empower healthcare professionals and contribute to more precise, efficient, and patient-centered care.